Lead-acid batteries feature a traditional design with lead plates submerged in an acid electrolyte, offering reliable performance and cost-effectiveness for battery pets. Calcium batteries improve upon this by incorporating calcium alloys in the plates, reducing water loss, enhancing corrosion resistance, and extending battery life. When choosing between lead-acid and calcium batteries, consider factors such as maintenance frequency, longevity, and overall durability to match your battery pet's power needs.
Table of Comparison
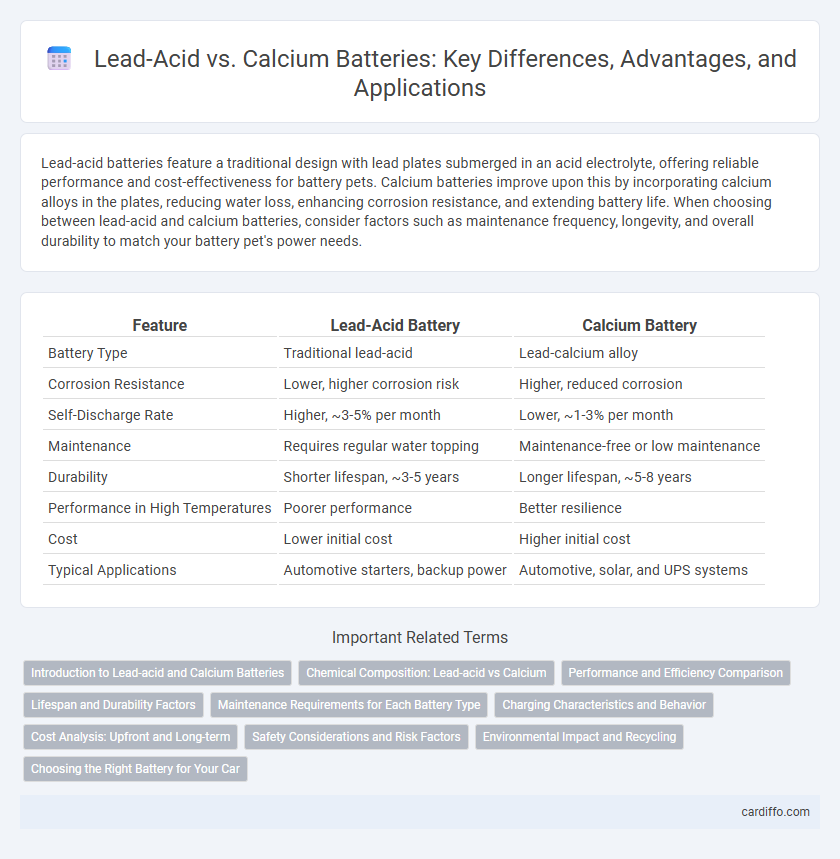
| Feature | Lead-Acid Battery | Calcium Battery |
|---|---|---|
| Battery Type | Traditional lead-acid | Lead-calcium alloy |
| Corrosion Resistance | Lower, higher corrosion risk | Higher, reduced corrosion |
| Self-Discharge Rate | Higher, ~3-5% per month | Lower, ~1-3% per month |
| Maintenance | Requires regular water topping | Maintenance-free or low maintenance |
| Durability | Shorter lifespan, ~3-5 years | Longer lifespan, ~5-8 years |
| Performance in High Temperatures | Poorer performance | Better resilience |
| Cost | Lower initial cost | Higher initial cost |
| Typical Applications | Automotive starters, backup power | Automotive, solar, and UPS systems |
Introduction to Lead-acid and Calcium Batteries
Lead-acid batteries utilize lead dioxide and sponge lead plates submerged in sulfuric acid electrolyte, offering reliable energy storage with high surge currents for automotive and backup power applications. Calcium batteries are a modified type of lead-acid battery incorporating calcium alloy plates, which reduce water loss, minimize self-discharge, and enhance corrosion resistance, improving overall durability. These advancements make calcium batteries more maintenance-free and longer-lasting compared to traditional lead-acid batteries.
Chemical Composition: Lead-acid vs Calcium
Lead-acid batteries utilize a lead dioxide cathode and sponge lead anode with sulfuric acid electrolyte, facilitating electrochemical reactions that generate electrical energy. Calcium batteries, a variant of lead-acid technology, incorporate calcium alloyed grids instead of pure lead, enhancing corrosion resistance and reducing water loss in the sulfuric acid electrolyte. The calcium addition modifies the battery's chemical composition, improving maintenance intervals and cycle life compared to traditional lead-acid cells.
Performance and Efficiency Comparison
Lead-acid batteries provide reliable energy storage with moderate cycle life and efficiency, while calcium batteries offer enhanced performance through reduced water loss and improved charge acceptance, resulting in longer service life and higher energy efficiency. Calcium batteries exhibit lower self-discharge rates and better resistance to corrosion, contributing to their superior durability under deep-cycle conditions. The overall efficiency of calcium batteries surpasses traditional lead-acid types by delivering faster recharge times and greater stability in extreme temperatures.
Lifespan and Durability Factors
Lead-acid batteries typically have a shorter lifespan of 3 to 5 years due to increased corrosion of the positive plate caused by the grid alloy. Calcium batteries offer enhanced durability and a longer lifespan ranging from 5 to 7 years, benefiting from reduced water loss and improved resistance to corrosion. The addition of calcium to the grid alloy minimizes water consumption and self-discharge rates, making calcium batteries more suitable for deep cycle applications and harsh environmental conditions.
Maintenance Requirements for Each Battery Type
Lead-acid batteries require regular maintenance such as checking electrolyte levels and topping up with distilled water to prevent sulfation and ensure optimal performance. Calcium batteries offer reduced maintenance needs due to their sealed design, which minimizes water loss and decreases the risk of corrosion. Choosing calcium batteries can lead to lower maintenance costs and longer service intervals compared to traditional lead-acid batteries.
Charging Characteristics and Behavior
Lead-acid batteries exhibit slower charging rates and higher self-discharge compared to calcium batteries, which benefit from lower water loss and reduced gassing during charging. Calcium batteries feature improved charge acceptance and enhanced resistance to overcharging, resulting in prolonged battery life and better maintenance intervals. Optimal charging voltage for lead-acid batteries typically ranges from 2.4 to 2.45 volts per cell, while calcium batteries require a slightly higher voltage around 2.45 to 2.5 volts per cell for efficient charging.
Cost Analysis: Upfront and Long-term
Lead-acid batteries generally have a lower upfront cost compared to calcium batteries, making them a popular choice for budget-conscious applications. Calcium batteries offer longer lifespan and reduced maintenance costs, which can lead to lower total cost of ownership over time despite their higher initial price. Evaluating the balance between initial investment and durability is essential for optimizing long-term cost efficiency in battery selection.
Safety Considerations and Risk Factors
Lead-acid batteries pose higher risks of acid spills and hydrogen gas explosions due to their liquid electrolyte, requiring careful ventilation and handling procedures to mitigate safety hazards. Calcium batteries, utilizing a solid or gel-like electrolyte, exhibit reduced gas emissions and lower maintenance needs, enhancing overall safety and minimizing corrosion risks in comparison. Both types demand proper charging protocols to prevent thermal runaway and extend battery lifespan while ensuring user safety.
Environmental Impact and Recycling
Lead-acid batteries contain toxic lead and sulfuric acid, posing significant environmental risks during disposal and recycling if not properly managed, while calcium batteries offer improved corrosion resistance, reducing electrolyte loss and extending battery lifespan, which can lower environmental impact. Both battery types require specialized recycling processes, but lead-acid battery recycling infrastructure is more established globally, recovering up to 99% of lead to prevent hazardous waste. Calcium batteries, though less common, demand advanced recycling techniques to safely reclaim materials and minimize ecological footprint, emphasizing the need for sustainable disposal practices in battery lifecycle management.
Choosing the Right Battery for Your Car
Lead-acid batteries are known for their reliability and cost-effectiveness, providing a stable power source suitable for standard automotive applications. Calcium batteries enhance performance with lower water loss, reduced self-discharge rates, and increased corrosion resistance, making them ideal for modern vehicles with higher energy demands. Selecting the right battery depends on factors such as climate, vehicle requirements, and maintenance preferences, where calcium batteries offer longer lifespan and better durability in extreme conditions.
Lead-acid vs Calcium Battery Infographic
 cardiffo.com
cardiffo.com