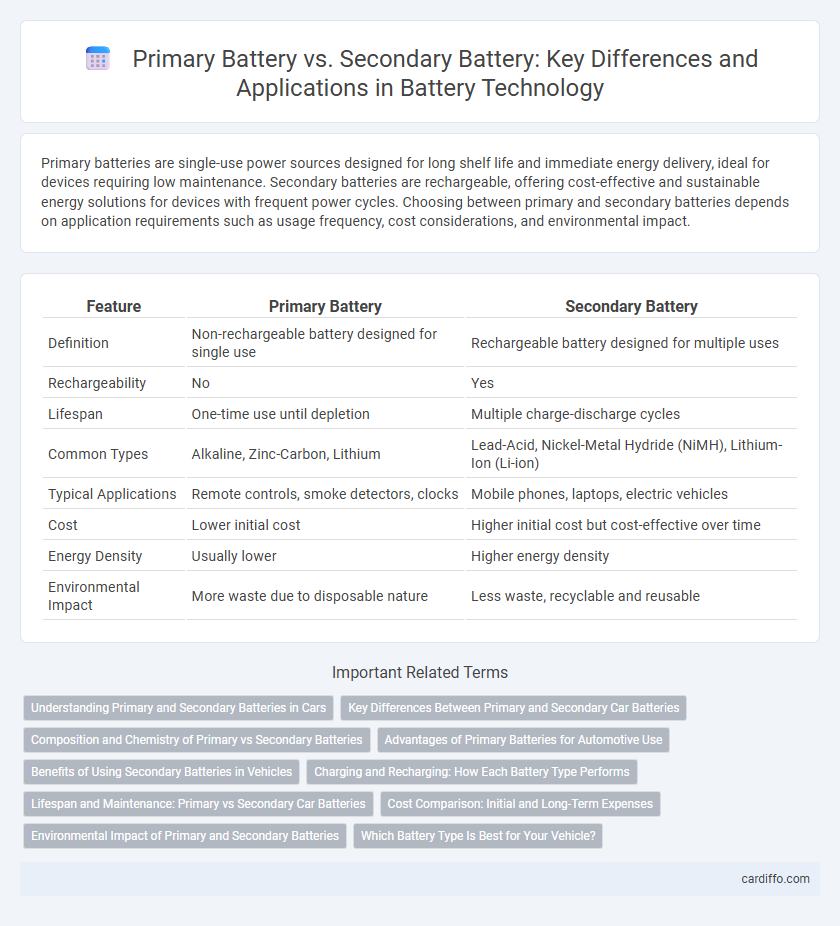
Primary batteries are single-use power sources designed for long shelf life and immediate energy delivery, ideal for devices requiring low maintenance. Secondary batteries are rechargeable, offering cost-effective and sustainable energy solutions for devices with frequent power cycles. Choosing between primary and secondary batteries depends on application requirements such as usage frequency, cost considerations, and environmental impact.
Table of Comparison
| Feature | Primary Battery | Secondary Battery |
|---|---|---|
| Definition | Non-rechargeable battery designed for single use | Rechargeable battery designed for multiple uses |
| Rechargeability | No | Yes |
| Lifespan | One-time use until depletion | Multiple charge-discharge cycles |
| Common Types | Alkaline, Zinc-Carbon, Lithium | Lead-Acid, Nickel-Metal Hydride (NiMH), Lithium-Ion (Li-ion) |
| Typical Applications | Remote controls, smoke detectors, clocks | Mobile phones, laptops, electric vehicles |
| Cost | Lower initial cost | Higher initial cost but cost-effective over time |
| Energy Density | Usually lower | Higher energy density |
| Environmental Impact | More waste due to disposable nature | Less waste, recyclable and reusable |
Understanding Primary and Secondary Batteries in Cars
Primary batteries in cars, also known as non-rechargeable batteries, are typically used for single-use applications such as remote key fobs and certain emergency devices due to their high energy density and long shelf life. Secondary batteries, or rechargeable batteries, power the main functions of vehicles, especially in electric and hybrid cars, by allowing repeated charging and discharging cycles that provide sustained energy over time. Understanding the distinction between primary and secondary batteries is crucial for optimizing vehicle performance, energy efficiency, and battery maintenance strategies.
Key Differences Between Primary and Secondary Car Batteries
Primary car batteries are non-rechargeable and designed for single-use applications, providing a steady voltage output until depletion, while secondary car batteries are rechargeable and support repeated charging cycles, making them ideal for vehicles requiring frequent energy replenishment. Primary batteries typically consist of alkaline or lithium chemistry, offering longer shelf life but limited lifespan, whereas secondary batteries use lead-acid or lithium-ion chemistries to enable energy storage and discharge through electrochemical reactions. The key differences lie in rechargeability, lifespan, cost-effectiveness, and suitability for automotive starting, lighting, and ignition (SLI) systems.
Composition and Chemistry of Primary vs Secondary Batteries
Primary batteries typically utilize non-rechargeable chemistries such as zinc-carbon or alkaline, where the anode and cathode materials undergo irreversible chemical reactions. Secondary batteries feature rechargeable chemistries, including lithium-ion, nickel-metal hydride (NiMH), and lead-acid, enabling reversible electrochemical processes through electrode material intercalation and redox reactions. The composition differences dictate energy storage mechanisms and lifecycle, with primary cells optimized for single-use and secondary cells engineered for multiple charge-discharge cycles.
Advantages of Primary Batteries for Automotive Use
Primary batteries offer significant advantages for automotive use due to their high energy density and long shelf life, enabling reliable performance without frequent recharging. They provide consistent power output in cold temperatures, ensuring dependable engine starts in various environmental conditions. Their maintenance-free nature reduces overall vehicle upkeep and enhances user convenience.
Benefits of Using Secondary Batteries in Vehicles
Secondary batteries in vehicles offer significant advantages due to their rechargeability, which leads to longer lifecycle and cost-effectiveness compared to primary batteries. These batteries support eco-friendly transportation by reducing waste and enabling energy regeneration during braking through regenerative braking systems. High energy density and reliable power output in secondary batteries enhance vehicle performance and contribute to sustainable mobility solutions.
Charging and Recharging: How Each Battery Type Performs
Primary batteries are designed for single-use and cannot be recharged, offering a stable voltage output until fully depleted. Secondary batteries support multiple charging cycles, allowing energy storage and reuse with gradual capacity loss over time. The performance during charging varies significantly; secondary batteries require controlled charging protocols to prevent damage, while primary batteries are unsuitable for recharging processes due to chemical design limitations.
Lifespan and Maintenance: Primary vs Secondary Car Batteries
Primary car batteries, designed for single-use, have a limited lifespan typically ranging from 3 to 5 years and require no maintenance due to their sealed construction. Secondary car batteries, also known as rechargeable or deep-cycle batteries, offer extended lifespans up to 7 to 10 years with proper maintenance, including regular charging and electrolyte level checks. The choice between primary and secondary batteries affects overall vehicle reliability and long-term cost efficiency, with secondary batteries favored for sustainable performance and reduced replacement frequency.
Cost Comparison: Initial and Long-Term Expenses
Primary batteries typically have a lower initial cost due to simple manufacturing and widespread availability, making them economical for single-use applications. Secondary batteries, though more expensive upfront because of complex materials and construction, offer significant long-term savings through rechargeability and extended lifecycle. Evaluating cost-effectiveness involves balancing the one-time expense of primary cells against the repeated use and reduced replacement frequency of secondary batteries.
Environmental Impact of Primary and Secondary Batteries
Primary batteries, typically single-use and non-rechargeable, contribute significantly to environmental pollution due to their frequent disposal and the release of toxic metals like mercury and cadmium. Secondary batteries, designed for multiple recharge cycles, reduce waste by extending the battery life and minimizing the need for raw material extraction. Recycling programs for secondary batteries further mitigate environmental impact by recovering valuable metals such as lithium, cobalt, and nickel.
Which Battery Type Is Best for Your Vehicle?
Primary batteries, such as alkaline or lithium cells, offer long shelf life and are ideal for emergency vehicle use where recharging isn't feasible. Secondary batteries, like lead-acid or lithium-ion, provide rechargeable power and are best suited for everyday vehicles requiring frequent starts and energy regeneration. Choosing the right battery depends on the vehicle's usage pattern, need for sustainability, and power demands.
Primary Battery vs Secondary Battery Infographic
 cardiffo.com
cardiffo.com