Isoparaffins offer superior stability and lower toxicity compared to aromatic hydrocarbons, making them ideal for cleaner fuel formulations. Aromatic hydrocarbons provide higher energy density and improved octane ratings but contribute to increased emissions and environmental concerns. Choosing between them depends on balancing performance requirements with environmental impact and regulatory compliance.
Table of Comparison
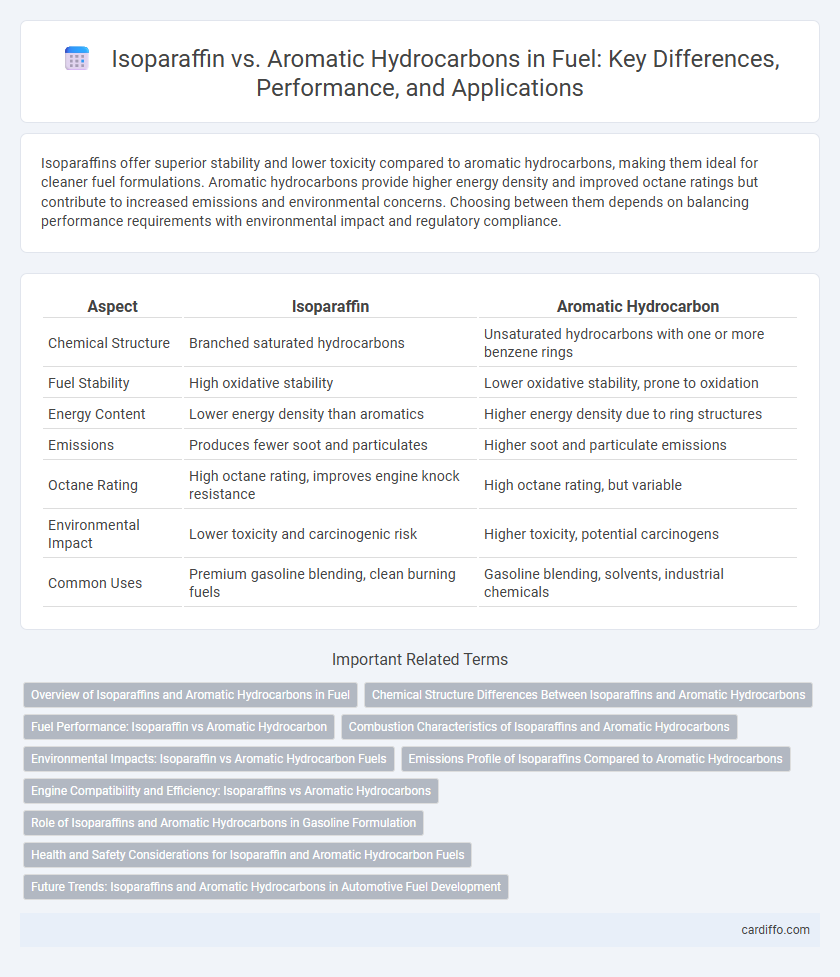
| Aspect | Isoparaffin | Aromatic Hydrocarbon |
|---|---|---|
| Chemical Structure | Branched saturated hydrocarbons | Unsaturated hydrocarbons with one or more benzene rings |
| Fuel Stability | High oxidative stability | Lower oxidative stability, prone to oxidation |
| Energy Content | Lower energy density than aromatics | Higher energy density due to ring structures |
| Emissions | Produces fewer soot and particulates | Higher soot and particulate emissions |
| Octane Rating | High octane rating, improves engine knock resistance | High octane rating, but variable |
| Environmental Impact | Lower toxicity and carcinogenic risk | Higher toxicity, potential carcinogens |
| Common Uses | Premium gasoline blending, clean burning fuels | Gasoline blending, solvents, industrial chemicals |
Overview of Isoparaffins and Aromatic Hydrocarbons in Fuel
Isoparaffins in fuel are branched-chain hydrocarbons known for their high octane ratings and low soot formation, making them ideal for cleaner combustion. Aromatic hydrocarbons, characterized by their ring-like structures, contribute to higher energy density but tend to increase emissions and particulate matter. Selecting between isoparaffins and aromatic hydrocarbons influences fuel performance, emissions, and engine durability in combustion applications.
Chemical Structure Differences Between Isoparaffins and Aromatic Hydrocarbons
Isoparaffins consist of branched-chain alkanes with saturated hydrocarbons featuring only single bonds, contributing to their high stability and low reactivity. Aromatic hydrocarbons contain one or more benzene rings with conjugated p-electron systems, which create enhanced chemical stability through resonance but also increased reactivity in electrophilic substitution reactions. The fundamental structural distinction lies in isoparaffins' saturated, non-aromatic framework versus the planar, cyclic, and unsaturated nature of aromatic hydrocarbons.
Fuel Performance: Isoparaffin vs Aromatic Hydrocarbon
Isoparaffins exhibit higher octane ratings and superior combustion efficiency compared to aromatic hydrocarbons, leading to enhanced engine performance and reduced knocking. Aromatic hydrocarbons, while providing higher energy density, tend to produce more soot and harmful emissions during combustion. The choice between isoparaffin and aromatic hydrocarbon fuels significantly impacts fuel stability, emissions profile, and engine longevity in modern combustion engines.
Combustion Characteristics of Isoparaffins and Aromatic Hydrocarbons
Isoparaffins exhibit higher octane numbers and cleaner combustion with lower soot formation compared to aromatic hydrocarbons, which tend to produce more particulate emissions due to their ring structures. The combustion of isoparaffins results in more complete oxidation and reduced engine deposits, enhancing fuel efficiency and engine longevity. Aromatic hydrocarbons, while offering high energy content, often cause increased unburned hydrocarbons and carbon monoxide emissions, affecting environmental performance.
Environmental Impacts: Isoparaffin vs Aromatic Hydrocarbon Fuels
Isoparaffin fuels exhibit significantly lower volatile organic compound (VOC) emissions compared to aromatic hydrocarbons, reducing ground-level ozone formation and smog. Aromatic hydrocarbons contribute to higher particulate matter and benzene derivatives, which are carcinogenic and impact air quality. The reduced toxicity and improved biodegradability of isoparaffins make them a more environmentally favorable option in fuel formulations.
Emissions Profile of Isoparaffins Compared to Aromatic Hydrocarbons
Isoparaffins exhibit a lower emissions profile compared to aromatic hydrocarbons due to their reduced aromatic content, which significantly decreases the formation of particulate matter and nitrogen oxides during combustion. The high octane rating and cleaner combustion properties of isoparaffins contribute to improved engine performance with fewer harmful exhaust emissions. Studies show that fuels rich in isoparaffins produce up to 30% less soot and lower overall greenhouse gas emissions than traditional aromatic hydrocarbon-based fuels.
Engine Compatibility and Efficiency: Isoparaffins vs Aromatic Hydrocarbons
Isoparaffins exhibit superior engine compatibility due to their high octane rating and clean combustion properties, reducing engine knocking and emissions. Aromatic hydrocarbons, while offering high energy content, often lead to increased soot formation and lower engine efficiency because of their complex molecular structure. Choosing isoparaffins over aromatics enhances fuel efficiency and engine longevity by promoting smoother combustion and minimizing deposit buildup.
Role of Isoparaffins and Aromatic Hydrocarbons in Gasoline Formulation
Isoparaffins enhance gasoline's octane rating and improve combustion stability by providing branched-chain hydrocarbons that resist knocking. Aromatic hydrocarbons contribute high octane values but increase emissions due to their higher soot formation and toxicity. Balancing isoparaffins and aromatics is critical in gasoline formulation to optimize engine performance while meeting environmental regulations.
Health and Safety Considerations for Isoparaffin and Aromatic Hydrocarbon Fuels
Isoparaffin fuels exhibit lower toxicity and reduced carcinogenic risks compared to aromatic hydrocarbons, making them safer for handling and exposure. Aromatic hydrocarbon fuels, such as benzene and toluene, pose significant health hazards including central nervous system depression and increased cancer risk, necessitating stringent safety protocols. Proper ventilation, use of personal protective equipment, and exposure monitoring are critical to mitigate health risks associated with aromatic hydrocarbons in fuel applications.
Future Trends: Isoparaffins and Aromatic Hydrocarbons in Automotive Fuel Development
Isoparaffins in automotive fuels are gaining traction due to their high octane numbers and cleaner combustion characteristics, which align with stricter emissions regulations. Aromatic hydrocarbons, despite their energy density advantages, face declining use because of their higher soot and pollutant formation. Future fuel formulations prioritize isoparaffins to enhance engine efficiency and reduce environmental impact, supported by advances in bio-based feedstocks and refining technologies.
Isoparaffin vs Aromatic Hydrocarbon Infographic
 cardiffo.com
cardiffo.com