Ethanol blends are commonly used as renewable fuel alternatives with lower emissions and improved engine performance due to their higher octane rating and compatibility with standard gasoline engines. Methanol blends, while offering superior combustion efficiency and lower production costs, require modifications to fuel systems because methanol is more corrosive and has a higher affinity for water absorption. Choosing between ethanol and methanol blends depends on vehicle compatibility, environmental impact goals, and availability of fueling infrastructure.
Table of Comparison
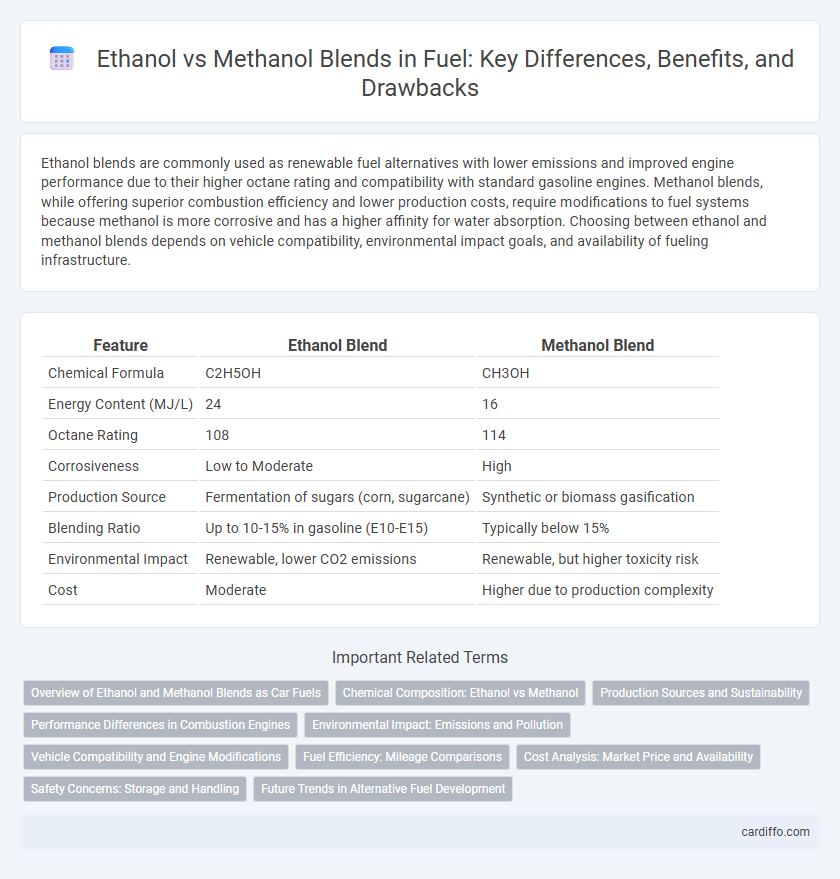
| Feature | Ethanol Blend | Methanol Blend |
|---|---|---|
| Chemical Formula | C2H5OH | CH3OH |
| Energy Content (MJ/L) | 24 | 16 |
| Octane Rating | 108 | 114 |
| Corrosiveness | Low to Moderate | High |
| Production Source | Fermentation of sugars (corn, sugarcane) | Synthetic or biomass gasification |
| Blending Ratio | Up to 10-15% in gasoline (E10-E15) | Typically below 15% |
| Environmental Impact | Renewable, lower CO2 emissions | Renewable, but higher toxicity risk |
| Cost | Moderate | Higher due to production complexity |
Overview of Ethanol and Methanol Blends as Car Fuels
Ethanol blends, typically mixed with gasoline at concentrations such as E10 or E85, provide renewable energy sources that reduce greenhouse gas emissions and enhance octane levels. Methanol blends, while less common, offer high octane ratings and can be produced from natural gas or biomass, but require engine modifications due to their corrosive properties and lower energy content. Both ethanol and methanol blends contribute to reducing reliance on fossil fuels, with ethanol being more widely adopted in flexible-fuel vehicles and methanol used primarily in specialized applications.
Chemical Composition: Ethanol vs Methanol
Ethanol, a two-carbon alcohol (C2H5OH), contains a hydroxyl group attached to an ethyl group, making it less toxic and more suitable for fuel blends compared to methanol, a one-carbon alcohol (CH3OH) with a simpler molecular structure. The presence of an additional carbon atom in ethanol enhances its energy content, offering approximately 30% more energy per gallon than methanol, which affects combustion efficiency and fuel economy. Methanol's higher oxygen content improves combustion emissions but its corrosive nature and toxicity require specialized materials for storage and handling in fuel systems.
Production Sources and Sustainability
Ethanol blends are predominantly produced from biomass sources such as corn, sugarcane, and cellulosic materials, making them more sustainable due to renewable feedstocks and lower greenhouse gas emissions. Methanol blends are primarily derived from natural gas and coal, though advancements in bio-methanol from biomass and CO2 capture offer improved sustainability prospects. The renewable origin of ethanol and emerging bio-methanol technologies position both fuels as viable alternatives with varying environmental impacts depending on feedstock and production methods.
Performance Differences in Combustion Engines
Ethanol blends typically provide higher octane ratings, improving engine efficiency and reducing knocking compared to methanol blends, which offer superior heat of vaporization that enhances cooling but may cause material corrosion. Methanol's lower energy content results in reduced fuel economy, whereas ethanol's higher energy density supports better mileage and power output in combustion engines. Engine components and fuel system compatibility must be considered, as methanol requires more robust materials to prevent degradation, influencing long-term performance and maintenance costs.
Environmental Impact: Emissions and Pollution
Ethanol blends typically produce lower greenhouse gas emissions compared to methanol blends due to their renewable biomass sources and higher energy content, which leads to more complete combustion and less carbon monoxide output. Methanol blends, derived often from natural gas or coal, tend to generate higher levels of formaldehyde and volatile organic compounds (VOCs), contributing to air pollution and smog formation. The overall environmental impact favors ethanol blends as they reduce fossil fuel dependency and lower particulate matter emissions, crucial for improving urban air quality.
Vehicle Compatibility and Engine Modifications
Ethanol blends, such as E10 and E85, are widely compatible with flex-fuel vehicles and require minimal engine modifications, making them suitable for most modern cars. Methanol blends, while offering high octane ratings, often demand significant engine adjustments, including modifications to fuel systems and seals, due to methanol's corrosive nature and lower energy content. Vehicle manufacturers typically recommend ethanol blends over methanol blends for standard internal combustion engines to ensure optimal performance and durability.
Fuel Efficiency: Mileage Comparisons
Ethanol blends typically offer higher fuel efficiency compared to methanol blends, with ethanol's energy content around 33% lower than pure gasoline but still superior to methanol, which has approximately 50% less energy per gallon. Vehicles running on ethanol blends generally achieve better mileage due to ethanol's favorable combustion properties and compatibility with existing engines. In contrast, methanol blends often result in reduced mileage and increased fuel consumption because of methanol's lower energy density and corrosive characteristics.
Cost Analysis: Market Price and Availability
Ethanol blends generally have a higher market price compared to methanol blends due to their widespread production and established supply chains. Methanol blends offer a cost advantage with lower raw material expenses and simpler production processes, making them more readily available in some regions. Availability of ethanol is closely tied to agricultural outputs, while methanol can be produced from natural gas or biomass, impacting regional pricing and supply stability.
Safety Concerns: Storage and Handling
Ethanol blends exhibit lower toxicity and reduced corrosiveness compared to methanol blends, making them safer for storage and handling in fuel applications. Methanol's higher volatility and toxicity require specialized containers and rigorous safety protocols to prevent inhalation hazards and leaks. Proper ventilation and use of compatible materials are critical to minimizing risks associated with methanol fuel storage and transportation.
Future Trends in Alternative Fuel Development
Ethanol blends dominate the alternative fuel market due to their compatibility with existing gasoline engines and renewable sourcing from biomass, ensuring substantial growth fueled by government mandates and carbon emission targets. Methanol blends, although less widespread, offer promising future potential because of their higher energy density and easier production from diverse feedstocks, including natural gas and CO2 capture technologies. Advances in catalyst development and fuel infrastructure are accelerating methanol's market adoption, positioning it as a viable complementary fuel in the transition to low-carbon energy systems.
Ethanol Blend vs Methanol Blend Infographic
 cardiffo.com
cardiffo.com