Battery pets with low self-discharge rates retain their charge longer when not in use, ensuring better performance over time. Charge acceptance measures how efficiently a battery pet can absorb and store energy during charging, directly impacting its overall lifespan and reliability. Optimizing both factors enhances the battery pet's efficiency, reducing maintenance and improving user satisfaction.
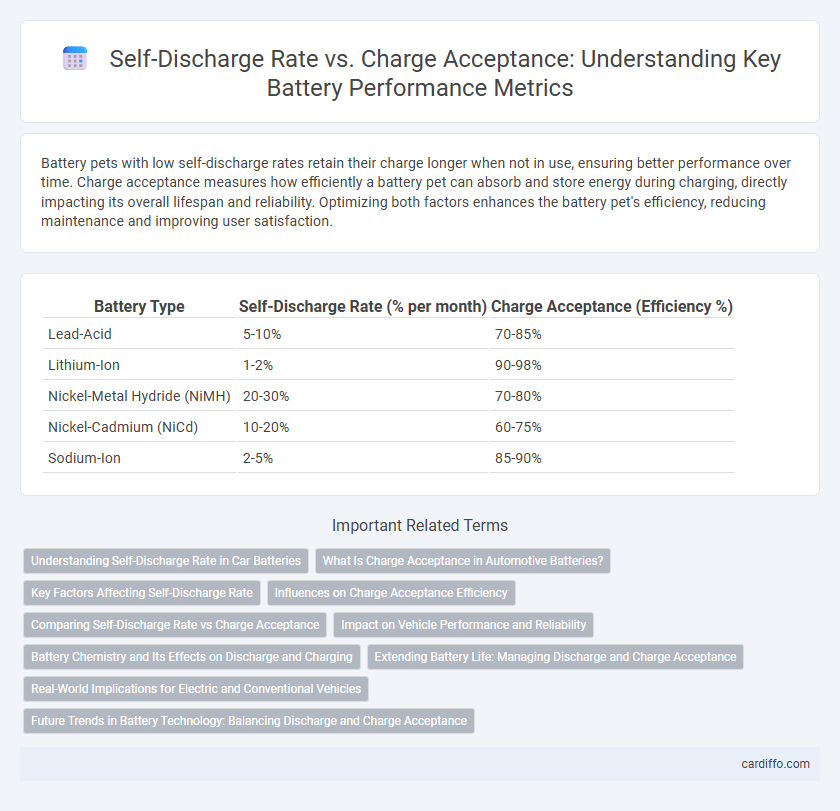
Table of Comparison
| Battery Type | Self-Discharge Rate (% per month) | Charge Acceptance (Efficiency %) |
|---|---|---|
| Lead-Acid | 5-10% | 70-85% |
| Lithium-Ion | 1-2% | 90-98% |
| Nickel-Metal Hydride (NiMH) | 20-30% | 70-80% |
| Nickel-Cadmium (NiCd) | 10-20% | 60-75% |
| Sodium-Ion | 2-5% | 85-90% |
Understanding Self-Discharge Rate in Car Batteries
Understanding the self-discharge rate in car batteries is crucial for maintaining optimal charge acceptance and prolonging battery life. This rate indicates how quickly a battery loses its stored charge when not in use, influenced by factors such as battery type, temperature, and age. A lower self-discharge rate enhances charge acceptance efficiency, ensuring reliable starting power and reducing the need for frequent recharging.
What Is Charge Acceptance in Automotive Batteries?
Charge acceptance in automotive batteries refers to the battery's ability to efficiently absorb and store electrical energy during the charging process, directly impacting vehicle performance and battery lifespan. It is influenced by factors such as battery chemistry, temperature, and state of charge, determining how quickly and effectively the battery can recover energy after use. A higher charge acceptance rate ensures faster recharging and reduces the impact of self-discharge, optimizing overall battery efficiency.
Key Factors Affecting Self-Discharge Rate
The self-discharge rate in batteries is primarily influenced by temperature, battery chemistry, and internal purity. Higher temperatures accelerate chemical reactions within the cell, increasing the self-discharge rate, while battery chemistries like lithium-ion exhibit lower self-discharge compared to nickel-based cells. Impurities and manufacturing defects in the electrode materials also contribute significantly to elevated self-discharge by creating unwanted side reactions.
Influences on Charge Acceptance Efficiency
The self-discharge rate significantly impacts charge acceptance efficiency by reducing the available capacity for energy storage, leading to diminished performance during charging cycles. Elevated self-discharge causes internal resistance increases and heat generation, which further lowers the battery's ability to accept charge efficiently. Temperature, battery chemistry, and state of health are critical factors influencing both self-discharge rates and subsequent charge acceptance characteristics.
Comparing Self-Discharge Rate vs Charge Acceptance
Batteries with low self-discharge rates retain stored energy longer, minimizing capacity loss during storage, while high charge acceptance enables faster energy absorption during charging cycles. Comparing self-discharge rate versus charge acceptance reveals a critical trade-off: batteries optimized for low self-discharge often exhibit slower charge acceptance due to chemical stability constraints, whereas those with high charge acceptance may experience increased self-discharge. Understanding this relationship helps in selecting battery types, such as lithium-ion or lead-acid, tailored to specific applications like electric vehicles or grid storage where either energy retention or rapid charging predominates.
Impact on Vehicle Performance and Reliability
A low self-discharge rate ensures longer battery life by minimizing energy loss during idle periods, directly improving vehicle reliability. High charge acceptance allows faster and more efficient energy replenishment, critical for maintaining optimal battery performance under various driving conditions. Balancing these factors is essential to enhance overall vehicle range, reduce downtime, and ensure consistent power availability.
Battery Chemistry and Its Effects on Discharge and Charging
Battery chemistry significantly influences self-discharge rates and charge acceptance, with lithium-ion cells exhibiting lower self-discharge compared to nickel-metal hydride (NiMH) or lead-acid batteries. Higher self-discharge rates in NiMH and lead-acid chemistries reduce effective charge retention, impairing overall energy efficiency and requiring more frequent charging cycles. Charge acceptance, or the battery's ability to absorb charge efficiently, is optimized in lithium-ion chemistries due to stable electrode materials and electrolyte composition, resulting in faster charging and prolonged battery lifespan.
Extending Battery Life: Managing Discharge and Charge Acceptance
Self-discharge rate significantly impacts battery longevity by causing energy loss even when the battery is idle, reducing overall charge acceptance over time. Optimizing charge acceptance involves controlling charging parameters to minimize stress and degradation, thus preserving capacity and extending battery life. Effective management of both self-discharge and charge acceptance enhances cycle life and maintains optimal performance in rechargeable batteries.
Real-World Implications for Electric and Conventional Vehicles
High self-discharge rates in batteries reduce charge acceptance, leading to shorter driving ranges and increased charging frequency for electric vehicles (EVs). Conventional vehicles using start-stop systems can experience reduced battery lifespan and reliability due to energy losses from self-discharge. Understanding these real-world implications helps optimize battery management systems, ensuring improved performance and durability in both EVs and traditional combustion engine vehicles.
Future Trends in Battery Technology: Balancing Discharge and Charge Acceptance
Future battery technologies aim to optimize self-discharge rates while enhancing charge acceptance to improve overall efficiency and lifespan. Advances in solid-state electrolytes and novel electrode materials contribute to reducing energy loss during storage and enabling faster, more efficient charging cycles. Integrating artificial intelligence for battery management systems further refines the balance between minimizing self-discharge and maximizing charge acceptance in next-generation energy storage solutions.
Self-Discharge Rate vs Charge Acceptance Infographic
 cardiffo.com
cardiffo.com